Research Success Stories
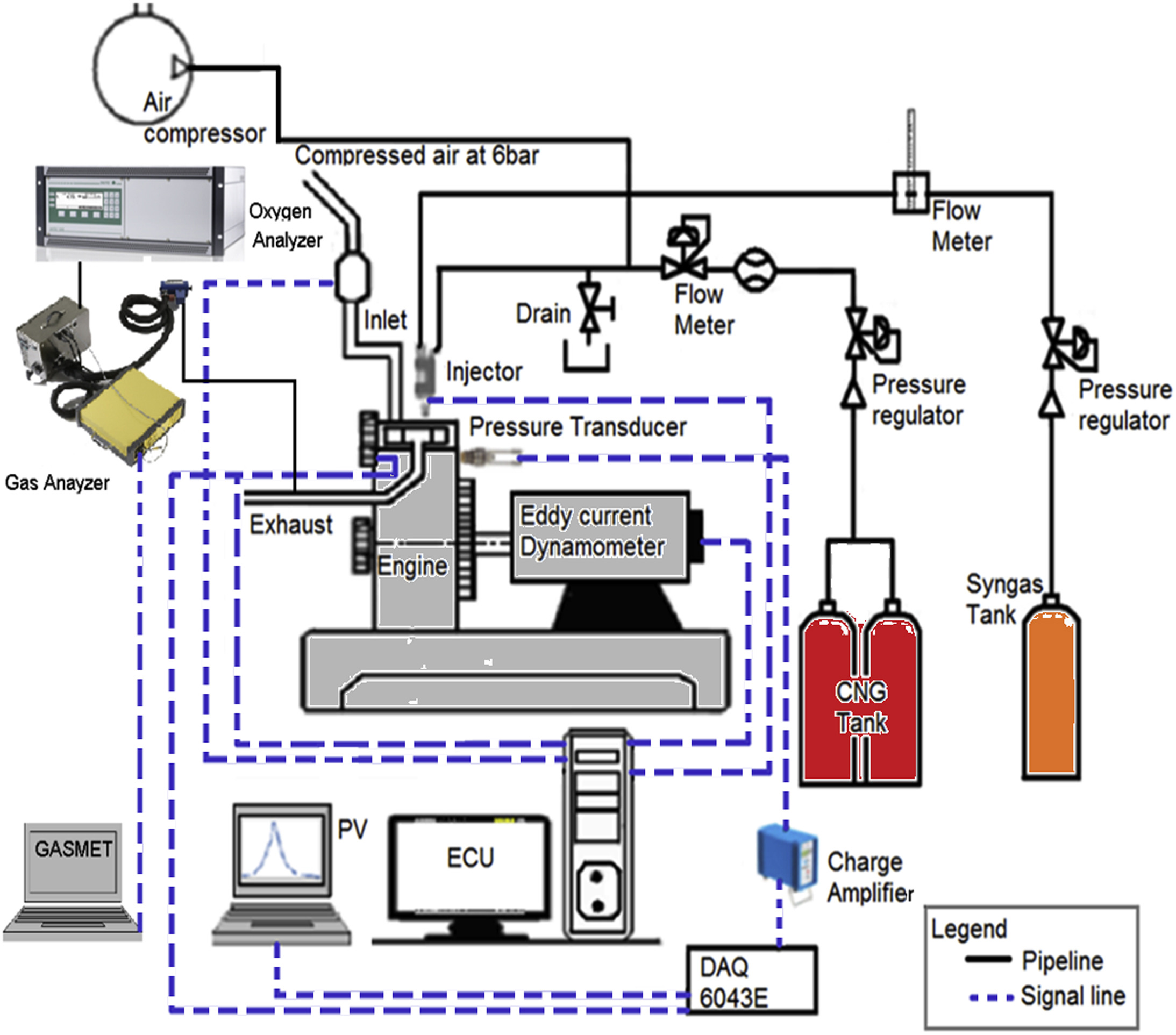
Syngas produced from gasification of solid fuels can serve best as transition fuel from the carbon-based to the hydrogen-based fuels in the internal combustion engines. The lone drawback is its low calorific value being between one tenth and one fifth of that of CNG (Compressed Natural Gas). This results in higher BSFC (brake specific fuel consumption) and limitation on the injection duration at late injection timings in the DI (direct-injection) SI (spark-ignition) engine. Recently, there have been efforts to enrich the syngas with methane so that the calorific value can be improved. This paper presents experimental results on the effect of methane-enrichment of syngas on the combustion, performance and emissions in the DISI engine. The result shows that the MES (methane-enriched syngas) has extended the operation excess air ratio (λ) compared to syngas and CNG at the same engine speed. Methane-enrichment has maintained the faster and smoother combustion, the lower brake emissions of carbon monoxide and total hydrocarbon, and higher brake emissions of nitrogen oxides observed with syngas. Besides, MES improved the maximum brake thermal efficiency and the BSFC of the syngas by 30.2% and 21.3%, respectively. Therefore, MES can be better replacement to CNG in the DISI engine at all load conditions.
Posted on: October 2015
Authored: Shaharin Anwar Sulaiman
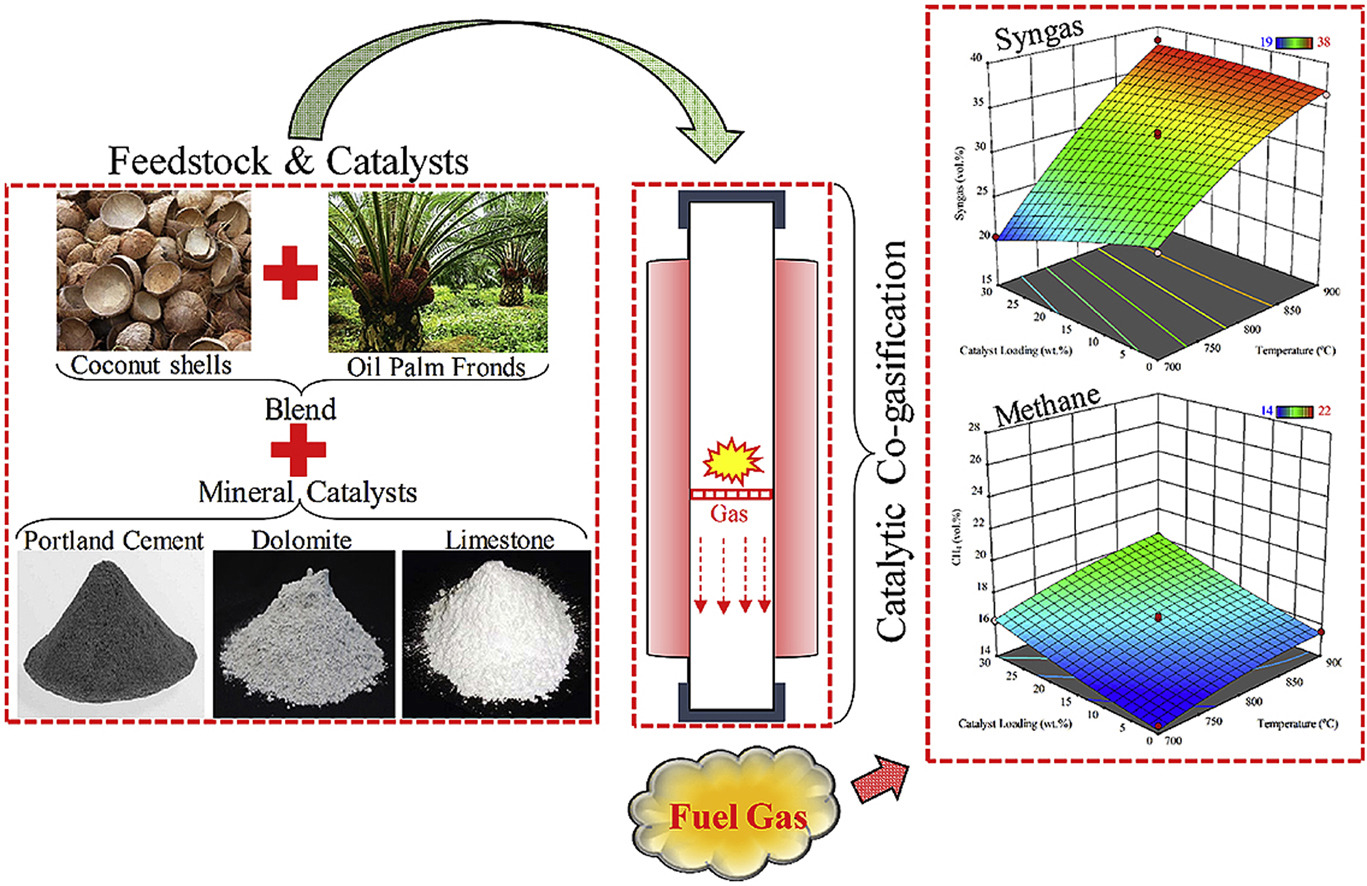
In this study, Response Surface Methodology (RSM) in combination with Box-Behnken Design (BBD) was used to optimize the temperature, catalyst loading, and blending ratio for a co-gasification process. The catalytic co-gasification of coconut shells (CS) and oil palm fronds (OPF) blends was performed in the presence of cement, dolomite, and limestone catalysts. A combined effect of temperature, catalyst loading, and blending ratio on production of H2, CO, and tar formation was investigated by using a BBD approach. The results showed the strongest influence of the process temperature on H2 and CO yield, and tar formation followed by the catalyst loading and blending ratio. A catalyst loading of 30 wt, process temperature of 900 °C and blending ratio of CS50:OPF50 were predicted as the optimized conditions for the reported co-gasification results. The highest H2 yield of 20.64 vol was produced during catalytic co-gasification of the blended biomass with limestone followed by the cement (18.22 vol) and dolomite (14.99 vol). Under optimized process conditions, lowest tar concentration of 0.87 g/Nm3 was obtained with limestone follow by the cement (1.42 g/Nm3) and dolomite (2.13 g/Nm3). However, blending ratio did not affect H2, CO yield, and tar formation appreciably. Conclusively, the mixing ratio of CS and OPF would have a negligible role in controlling the process output.
Posted on: August 2019
Authored: Shaharin Anwar Sulaiman
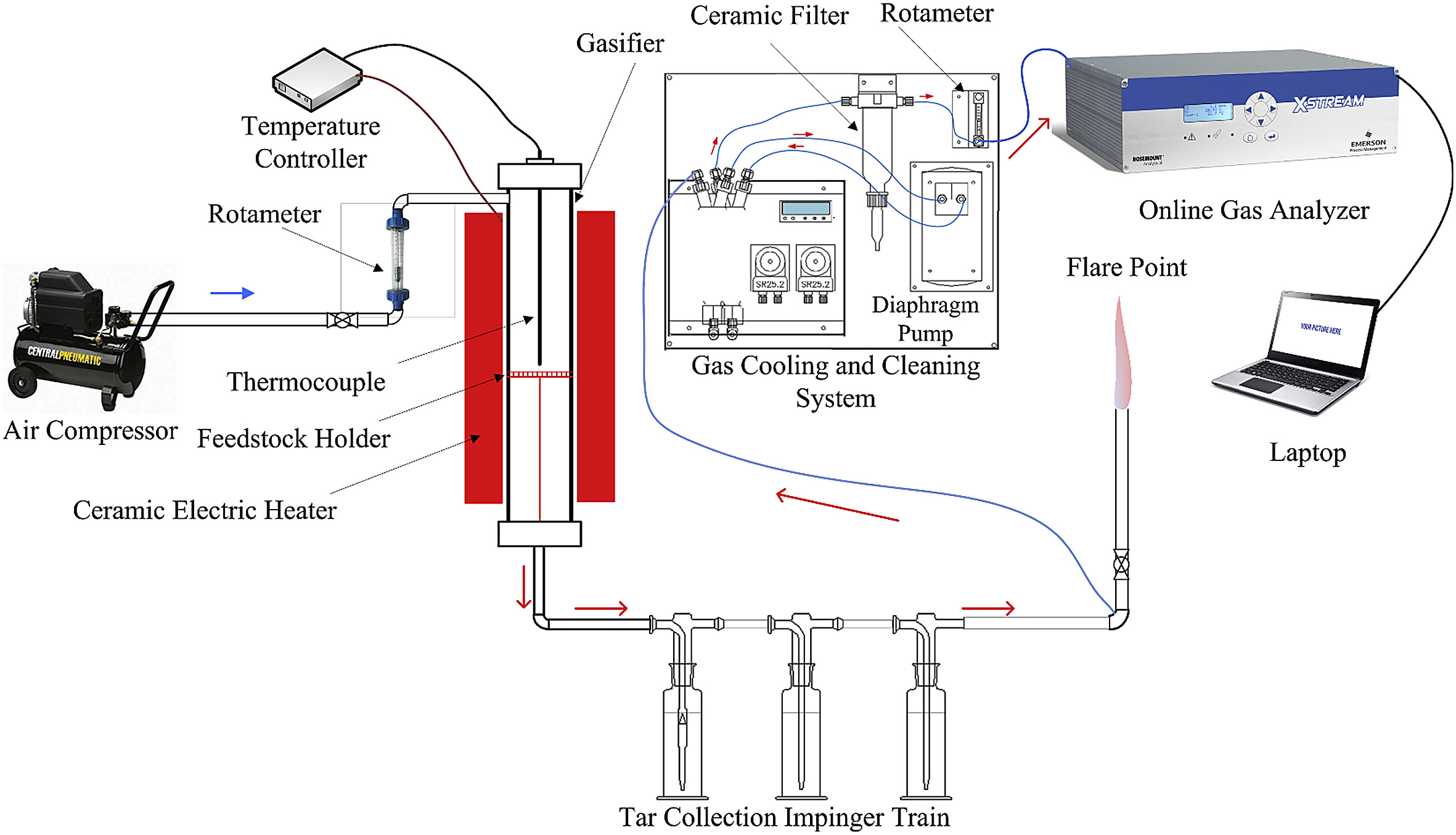
Palm kernel shell (PKS) biomass has great potential for power generation via gasification as it contains high energy content. However, abundant it may be, the source of PKS is scattered throughout the country, thus the consistency of feedstock supply may be hard to maintain. Co-gasifying with another source, such as plastics, can be seen as one of a solution to mitigate the supply chain problem. Polystyrene (PS) plastics have potential as a plastic feedstock because of its high domestic and industrial usage. As PS is also hard to recycle, using PS as a co feedstock for gasification is a way for PS waste management. However, the study on the performance of air co-gasification of PKS and PS has not been done before. It is essential to investigate the performance before it is utilized in the real world. In this work, the performance co-gasification of PKS and PS with different operating conditions was investigated. The gasification experiment was done in an electrically heated downdraft gasifier with a diameter of 8 cm. The reaction temperature was varied from 700 to 900 °C, with the equivalence ratio varied from 0.07 to 0.27. The PS weight percentage of the total feedstock was varied from 0 to 30 wt%. It was found that the vol% of CO and H2 on the producer gas increased with temperature while reducing the vol% of CO2 and CH4. HHV and the amount of gas produced were also increasing with increasing temperature. Increasing ER reduced the HHV of the gas but increased the amount of gas produced. Adding more PS to the feedstock blend increased the percentage of the produced gas at 900 °C, however, at the lower temperature of 800 °C, the percentage of gas produced decreased with increasing PS wt%.
Posted on: June 2020
Authored: Shaharin Anwar Sulaiman
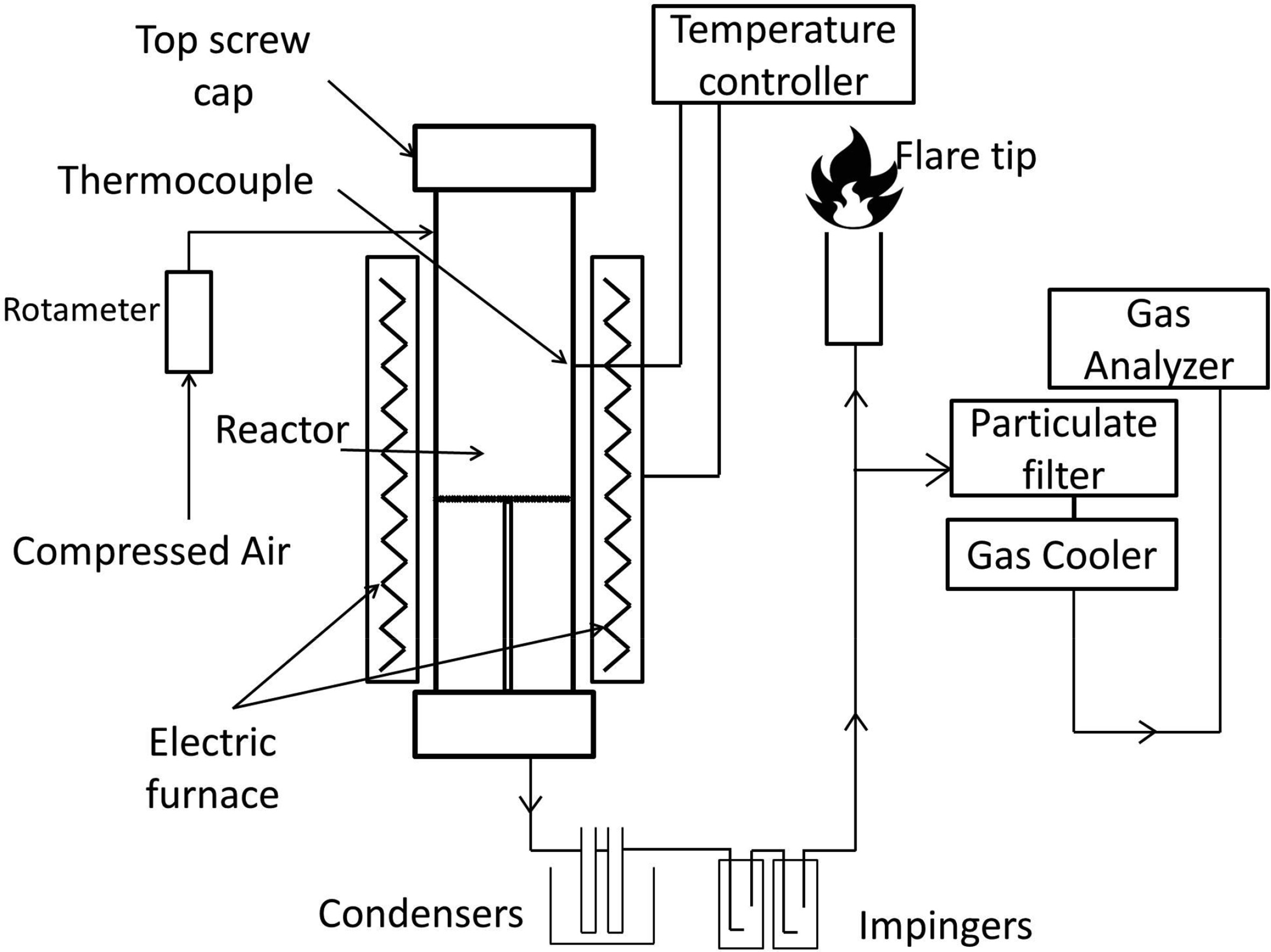
Biomass gasification is a promising approach for bioenergy conversion. Usually, biomass gasification is facing interruption in feedstock supply due to seasonal availability of biomass. In biomass gasification, formation of tar also affects the gasification efficiency. Therefore, in this study, catalytic air co-gasification of two palm wastes (coconut shells; CS, oil palm fronds; OPF) was investigated for syngas (H2+CO) and methane production in downdraft gasifier using three mineral catalysts such as Portland cement, dolomite, and limestone to address the issues. The three main process variables were investigated within the specific range, the temperature of 700-900 °C, catalyst loading of 0-30 wt%, and the biomass blending ratio of 20-80 wt%. Response Surface Methodology, Box-Behnken Design was used for process optimization. The results showed that temperature was the most influencing parameter for syngas production, followed by catalyst loading and blending ratio. The maximum methane produced from Portland cement catalyst followed by limestone and dolomite. The syngas and methane yield was obtained 38.81 vol% and 19.96 vol% respectively at optimized conditions of catalyst loading of 20 wt%, temperature of 900 °C, and blending ratio of CS20:OPF80 using Portland cement as a catalyst. The higher syngas and methane yields from catalytic co-gasification as compared to non-catalyst co-gasification was due to the catalytic effect of Ca, Fe, Mg, K, P, and Al oxides present in catalysts and biomass materials.
Posted on: January 2020
Authored: Shaharin Anwar Sulaiman
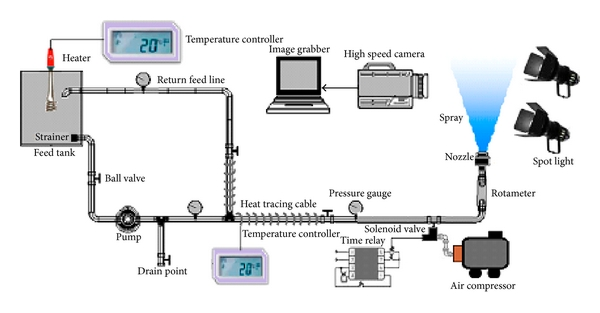
The objective of the research was to understand and improve the unusual physical and atomization properties of the complexes/adhesives derived from the tapioca starch by addition of borate and urea. The characterization of physical properties of the synthesized adhesives was carried out by determining the effect of temperature, shear rate, and mass concentration of thickener/stabilizer on the complex viscosity, density, and surface tension. In later stage, phenomenological analyses of spray jet breakup of heated complexes were performed in still air. Using a high speed digital camera, the jet breakup dynamics were visualized as a function of the system input parameters. The further analysis of the grabbed images confirmed the strong influence of the input processing parameters on full cone spray patternation. It was also predicted that the heated starch adhesive solutions generate a dispersed spray pattern by utilizing the partial evaporation of the spraying medium. Below 40°C of heating temperature, the radial spray cone width and angle did not vary significantly with increasing Reynolds and Weber numbers at early injection phases leading to increased macroscopic spray propagation. The discharge coefficient, mean flow rate, and mean flow velocity were significantly influenced by the load pressure but less affected by the temperature.
Posted on: January 2014
Authored: Shaharin Anwar Sulaiman