Research Success Stories
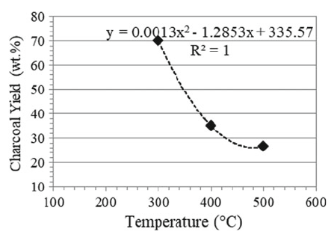
Charcoal, a black carbon residue, is mostly produced from the major conventional method where the biomass is allowed to be heated for several days in a kiln without studying the process condition. Most of the studies on the pyrolysis process focus on the liquid and gaseous by-products neglecting the solid to be used as a combustion fuel. For this study, charcoal was produced from coconut shells by the thermochemical conversion method of pyrolysis in a controlled nitrogen environment at temperatures of 300 Celcius, 400 Celcius, and 500 Celcius, and residence times of 15 min, 30 min, and 60 min. This was conducted to evaluate the process conditions effects concerning the charcoal calorific value and yield. From the results obtained, a high process condition increases the calorific value, which results in a decrease in the charcoal yield. The lowest temperature gives a yield of 70.18 wt% and calorific value of 25.30 MJ/kg while the highest temperature produces a yield for as low as 26.57 wt% and a high calorific value of 30.15 MJ/kg. Furthermore, the charcoal yield tends to decrease from 51.99 to 33.10 wt% and the calorific value increases as the residence time increases from 15 to 45 min. Consequently, the thermal conversion undergone by the biomass may cause the changes of the quality parameters. Thus, charcoal can replace the use of fossil fuels because it presents energy content higher than that of lignite and similar to that of coal.
Posted on: September 2020
Authored:Shaharin Anwar Sulaiman
The world does not have too much time to ensure that the fast-growing population has enough land, food, water and energy. The rising food demand has brought a positive surge in fertilizers' demand and agriculture-based economy. The world is using 170 million tons of fertilizer every year for food, fuel, fiber, and feed. The nitrogenous fertilizers are being used to meet 48% of the total food demand of the world. High fertilizer inputs augment the reactive nitrogen levels in soil, air, and water. The unassimilated reactive nitrogen changes into a pollutant and harms the natural resources. The use of controlled-release fertilizers for slowing down the nutrients' leaching has recently been practiced by farmers. However, to date, monitoring of the complete discharge time and discharge rate of controlled released fertilizers is not completely understood by the researchers. In this work, corn starch was thermally processed into a week gel-like coating material by reacting with urea and borate. The granular urea was coated with native and processed starch in a fluidized bed reactor having bottom-up fluid delivery system. The processed starch exhibited better thermal and mechanical stability as compared to the native starch. Unlike the pure starch, the storage modulus of the processed starch dominated the loss modulus. The release time of urea, coated with processed starch, remained remarkably larger than the uncoated urea.
Posted on: April 2020
Authored:Shaharin Anwar Sulaiman
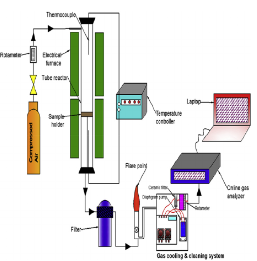
Coconut shell charcoal is an important product obtained from coconut shells, which are widely used as domestic and industrial fuel. Wastes of coconut residues (shells, husks and coir) from processing of coconut milk and oil industries are widely generated everywhere all over the world. They have the potential of use as a fuel in renewable energy for different applications. In this study, the production of charcoal from coconut shell through the process of pyrolysis was conducted to determine the influence of particle size, temperature and residence time on the charcoal weight loss. Pyrolysis is a thermochemical conversion method which converts biomass into more valuable products in form of solid (charcoal, bio-char and activated carbon), liquid (oil) and gas in the absence of oxygen. The process was carried out under various process conditions of temperature (300 Celcius, 400 Celcius, and 500 Celcius), residence time (15 minutes, 30 minutes, and 60 minutes), and particle size (5.0 mm and 25.0 mm). It was found that for both the temperature and the residence time, the weight loss from the coconut shell increase linearly with increase in temperature and residence time. The particle size has influence in percentage weight loss. The resulting charcoal obtained can be utilized as an alternative energy resource for different applications.
Posted on:June 2020
Authored:Shaharin Anwar Sulaiman
The rate of oil palm production in Malaysia increases annually and as a result, the oil palm wastes, especially oil palm trunk (OPT) and oil palm fronds (OPF) remain abundant. A suitable way of converting this abundant waste to renewable energy is through thermochemical conversion. Thus, this study investigates the characteristics of OPT and OPF biomass, for use as feedstock in thermochemical processes like gasification, pyrolysis, and combustion. The analysis carried out includes; ultimate (CHNSO) and proximate (thermogravimetric) analysis, calorific value, field emission scanning electron microscopy (FESEM) and x-ray fluorescence (XRF). Both feedstocks exhibited potential for use as fuel in biomass thermochemical conversion. The CHNSO analysis showed the presence of sufficient carbon, hydrogen and oxygen elements in both feedstocks, with carbon being the highest 45.42% in OPT and 43.35% in OPF. The percentages of nitrogen and sulphur which are required to be less for a good fuel were also obtained in low quantities for both fuel; 0.47% and 0.13% in OPT and 0.76% and 0.45% in OPF, respectively. The thermogravimetric analysis revealed both feedstocks to be having high volatile matter 62.28% in OPT and 66.10% in OPF. Meanwhile, sufficient fixed carbon content of 26.18% in OPT and 25.68% in OPF with low ash content of 9.82% in OPT and 6.32% in OPF were obtained in the analysis. FESEM and XRF were used to investigate the surface morphology, elemental and mineralogical nature of the samples. The findings were compared with those of other biomass and non-biomass materials. The EDX graph showed the presence of carbon and oxygen in a higher amount while in the XRF analysis CaO and K2O were the major oxides present in both OPT and OPF, with a low amount of SiO making the feedstocks less prone to agglomeration during thermochemical conversion.
Posted on:June 2020
Authored:Shaharin Anwar Sulaiman
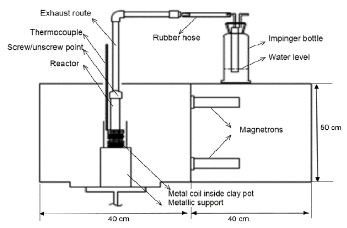
Rapid rise in production of plastic waste has posed a threat to environment due to its nonbiodegradable nature. In recent years, microwave assisted pyrolysis of plastic waste has emerged as a promising solution toward reduction of waste and recovery of value-added products and fuels. Previous works on microwave-metal interaction pyrolysis estimated only the peak value of coil temperature at a constant microwave power without monitoring complete reaction. The current study was directed toward investigating the effect of reaction time and variable microwave power on the coil temperature during microwave-metal interaction pyrolysis of plastics. The experiment was performed on individual plastics PS (polystyrene), PP (polypropylene) and LDPE (low density polyethylene) for comparative analysis. Pyrolysis was carried out for a reaction time of 30 minutes at different values of microwave power in the range of 500-2500 W. Type K thermocouple was used to monitor temperature of metal coil. Fluctuation in temperature of coil was found to be a consequence of interaction of coil and thermocouple with the microwaves. Maximum heating rate was observed in the first 5 minutes of microwave exposure. The slopes of average temperature versus microwave power were found to be nearly equal estimated as: PS = 0.24 Celcius/W, PP = 0.20 Celcius/W, LDPE = 0.18 Celcius/W, which indicated consistency in heating process for each plastic, achieved by the current set-up. Further, it was inferred that nature of plastic pyrolyzed had insignificant influence on coil temperature due to absence of direct contact between plastic and the coil.
Posted on:June 2020
Authored: Shaharin Anwar Sulaiman