Mechanistic Approaches of Internalization, Subcellular Trafficking, and Cytotoxicity of Nanoparticles for Targeting the Small Intestine
Author: Faiz Ahmad- 2021
Asadullah Madni, Sadia Rehman, Humaira Sultan, Muhammad Muzamil Khan, M. Rafi Raza, Nadia Rai & Farzana Parveen
Abstract
Targeting the small intestine employing nanotechnology has proved to be a more effective way for site-specific drug delivery. The drug targeting to the small intestine can be achieved via nanoparticles for its optimum bioavailability within the systemic circulation. The small intestine is a remarkable candidate for localized drug delivery. The intestine has its unique properties. It has a less harsh environment than the stomach, provides comparatively more retention time, and possesses a greater surface area than other parts of the gastrointestinal tract. This review focuses on elaborating the intestinal barriers and approaches to overcome these barriers for internalizing nanoparticles and adopting different cellular trafficking pathways. We have discussed various factors that contribute to nanocarriers’ cellular uptake, including their surface chemistry, surface morphology, and functionalization of nanoparticles. Furthermore, the fate of nanoparticles after their uptake at cellular and subcellular levels is also briefly explained. Finally, we have delineated the strategies that are adopted to determine the cytotoxicity of nanoparticles.s
Methodology
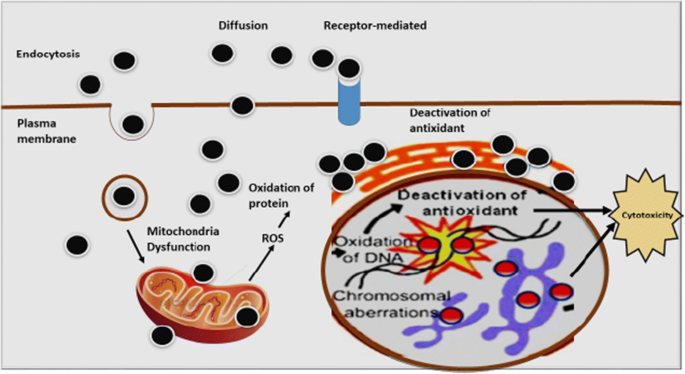
The methodology employed in this review involved a comprehensive analysis of existing literature concerning the internalization, intracellular interactions, cytotoxicity, and toxicity assessment methods associated with nanoparticles. Initially, a thorough search of scholarly databases such as PubMed, Scopus, and Web of Science was conducted to identify relevant articles published in peer-reviewed journals. Data extracted from these sources were then systematically synthesized to provide a detailed overview of various aspects, including the mechanisms involved in nanoparticle internalization across intestinal barriers such as the mucus layer, epithelial layer, and tight junctions. Additionally, the influence of physical and chemical nanoparticle properties on internalization and subsequent cellular and subcellular fate was meticulously examined. The review highlighted the heightened toxicity of nanoparticles compared to micrometer-sized particles, emphasizing the importance of studying their cytotoxic responses for the development of optimized nanodelivery systems. Moreover, the impact of nanoparticle size on cytotoxicity was explored, with smaller nanoparticles shown to interact more extensively with cellular membranes, potentially leading to increased cytotoxic effects. Furthermore, the review delved into the role of nanoparticle shape in influencing internalization processes such as endocytosis and phagocytosis, with spherical nanoparticles demonstrated to exhibit faster endocytosis than tubular counterparts. Future prospects in the medical field were discussed, underscoring the diverse applications of nanoparticles in areas such as cancer treatment, autoimmune disorders, catalysis, drug delivery, and electronics. Finally, the review examined the mechanisms by which nanoparticles may attach to biological membranes, inducing cytotoxic effects through the production of reactive oxygen species and subsequent cellular damage, ultimately leading to cell death. Through this methodological approach, the review provided valuable insights into the multifaceted interactions between nanoparticles and biological systems, paving the way for further research and advancements in nanoparticle-based technologies.
Impact & Benefits
Enhanced Therapeutic Efficacy: Understanding the factors influencing nanoparticle internalization allows for the optimization of drug delivery systems, leading to improved therapeutic outcomes. By modifying nanoparticle properties such as size, surface charge, and shape, researchers can enhance cellular uptake and target specific sites of action within the body, potentially increasing the efficacy of therapeutic agents while minimizing side effects.
Minimization of Side Effects: Tailoring nanoparticle properties to optimize internalization can help minimize off-target effects and reduce the risk of adverse reactions in patients. By enhancing cellular uptake and targeting specific tissues or cells, nanoparticles can deliver therapeutic agents more selectively, thereby minimizing damage to healthy tissues and organs.
Advancements in Drug Delivery: Functionalizing nanoparticles with various proteins or ligands allows for targeted drug delivery, enabling specific interactions with cellular receptors or transport mechanisms. This approach can enhance the rate of transcytosis across epithelial barriers, leading to improved bioavailability and therapeutic efficacy of orally administered drugs.
Innovations in Biomedical Research: The methodologies described pave the way for continued innovation in biomedical research, particularly in the fields of drug delivery, diagnostics, and therapeutics. By elucidating the mechanisms of nanoparticle internalization and functionalization, researchers can develop novel strategies for addressing complex biomedical challenges and improving patient care.
Market Potential
Nanoparticles offer versatile solutions in drug delivery, diagnostics, and therapeutics, with their unique properties enabling targeted delivery, enhanced efficacy, and reduced cytotoxicity. As research continues to advance, nanoparticles are poised to play an increasingly significant role in catalysis, medicine, antimicrobials, biosensors, drug delivery, and electronics. Their ability to interact with biological systems opens up opportunities for innovation in areas such as cancer treatment, autoimmune disorders, and environmental remediation, driving growth in the nanoparticle market.