Modification of bauxite residue with oxalic acid for improved performance in intumescent coatings
Author: Faiz Ahmad - June 2021
Adiat I. Arogundade, Puteri S.M. Megat-Yusoff, Faiz Ahmad, Aamir H. Bhat, Lukmon O. Afolabi
Abstract
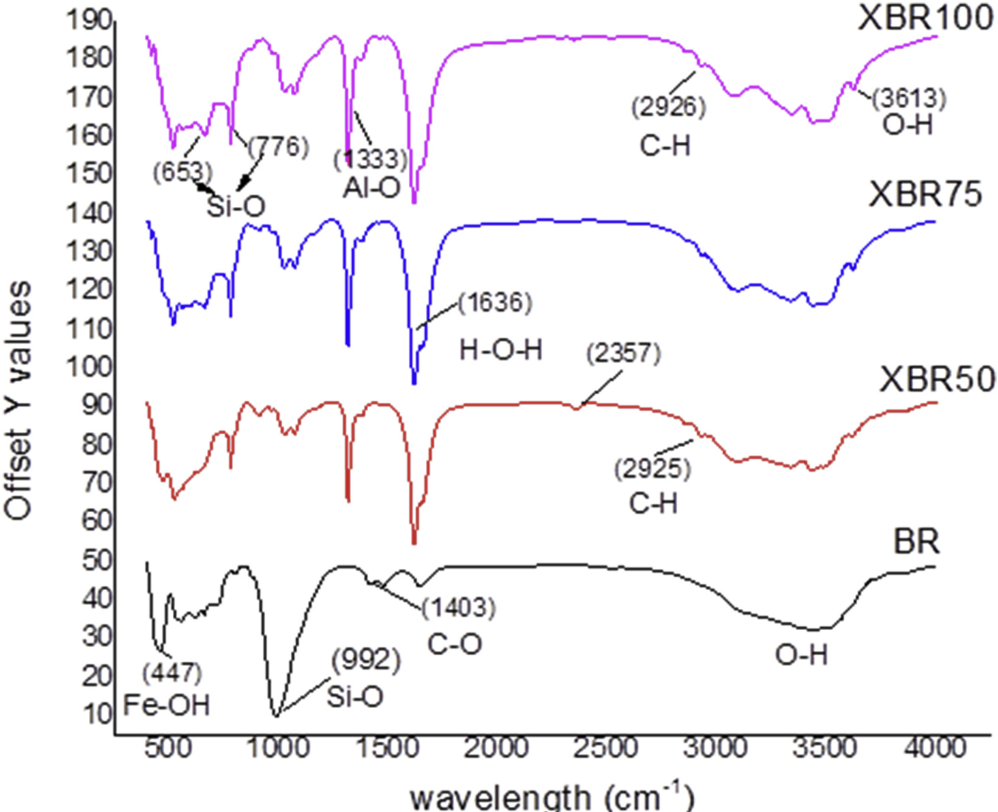
Valorization of bauxite residue (BR) enhances the dynamics of its application in intumescent coating for fire retarding systems. This BR, an alumina production waste could contain up to 45% ferrous oxide along with residual aluminous minerals. In an attempt to optimize the fire retardant properties of these minerals in intumescent systems, BR was treated in oxalic acid, varying the heating temperature between 50°C and 100°C at a constant pH of 2.65. X-ray florescence spectrometry revealed up to 80% reduction in iron content and total dissolution of desilication products (DSPs). The process temperature was found to affect the efficiency with which iron oxide was removed and with which the dissolved DSPs were precipitated as aluminum hydrates. X-ray diffraction revealed increased crystallinity and a gibbsite-dominated compound. Incorporation of the modified bauxite residues into a control intumescing formulation resulted in improved endothermic cooling, increased char expansion and char reinforcement. An inverse relationship appeared to exist between aluminum hydrates and iron as removal of iron led to enhanced intumescence and increased char expansion while higher iron content led to a compact, less expanded char. A balance of the fire retarding minerals occurred at a leaching temperature of 75°C in oxalic acid. Best heat shielding performance thus occurred at XBR75-IC5 as char expansion increased by 12% and the substrate temperature reduced by 31% over the control IC system. Thus, BR may act as alternative fire retardant filler for intumescing systems.
Methodology
The bauxite residue was obtained from Virotec, Australia. Oxalic acid dihydrate (Merck), Iron II Sulphate and ammonium hydroxide were purchased from Avantis, Sdn. Bhd. Distilled water was used throughout the hydrothermal process. Ammonium polyphosphate, APP Exolit 42 was purchased from Clariant Sdn. Bhd., Malaysia, Expandable graphite, Melamine and Boric acid were supplied by Sigma-Aldrich, Sdn. Bhd. Malaysia; the resin, epoxy BE-118 and hardener, polyamide amine, ACR H-2310 were purchased from Mc Growth Sdn. Bhd. Malaysia.
Impact & Benefits
Improved Fire Resistance:BR can be used as a filler in intumescent coatings, which are designed to provide fire protection by expanding and forming an insulative barrier when exposed to high temperatures.
Enhanced Char Strength:The incorporation of BR can improve the structural integrity of the char formed during fire exposure. The presence of iron oxides in BR aids in the graphitization of the char, making it more compact and resistant to mechanical stresses
Waste Utilization:Utilizing BR in intumescent coatings helps in the disposal of this industrial waste, addressing a significant environmental challenge.
Reduction of Hazardous Material:The hydrothermal treatment of BR with oxalic acid not only enhances its fire retardant properties but also reduces its iron oxide content, mitigating potential environmental hazards associated with its disposal.
Cost-Effective Filler: The use of BR can lower the production costs of intumescent coatings, making fire protection more affordable.
Enhanced Performance: Studies have shown that optimizing the treatment of BR can lead to improved performance of intumescent coatings. For example, leaching BR in oxalic acid at optimal temperatures enhances char expansion and reduces substrate temperature during fire exposure.
Versatility: BR can be used in both modified and unmodified states, offering flexibility in formulation and application. This versatility allows for the fine-tuning of intumescent coatings to meet specific fire protection requirements.
Findings/Figures and Research Data
The char micrographs in Fig. 6 clearly illustrate the effect of modification with BR on the intumescent system. The control char in Fig. 6a appeared weak, shrivelled and torn due to long exposure (60 min) to the butane flame at a temperature of 900 °C + 100 °C. The discontinuous, open surface allowed easy infiltration of heat to the underlying steel substrate, leading to a rapid rise in substrate temperature. Fig. 6b in comparison, revealed a closed continuous surface. The unmodified BR-reinforced char displayed better oxidative resistance to the impacting flame due to the added effects of the fire retardant minerals contained in bauxite residue. The fire retardant minerals in BR include CaCO3 which decomposes between 600 °C to 750 °C to release CO2, a common flame retarding gas. Other major fire retardant minerals are the metal hydrates, gibbsites and goethite which dehydrate between 250 °C to 350 °C to release water vapour [44]. Titanium oxide (anatase) and the metal oxides generated after dehydroxylation add mass to the char and form the bulk of the char long after the more oxidative content of the char has burnt off.
Market Potential
Fire Retardant Properties:BR contains residual aluminous hydrates and other compounds that enhance fire retardant properties when used as fillers in intumescent systems.
Char Reinforcement: BR can reinforce the char, providing mechanical strength and stability against perturbations from mechanical forces and thermal stress.
Waste Reduction:The incorporation of BR into intumescent coatings can help mitigate the environmental problem of BR disposal, potentially reducing the annual generation of BR waste.
Optimization:Further research and development are needed to optimize the formulation to increase the BR content in the coatings while maintaining or enhancing performance.
Market Acceptance:Gaining market acceptance will require demonstrating the performance benefits and cost advantages of BR-based intumescent coatings compared to traditional options.