Visualizing Hyperactivation in Neurodegeneration Based on Prefrontal Oxygenation: A Comparative Study of Mild Alzheimer's Disease, Mild Cognitive Impairment, and Healthy Controls
Author: Tang Toong Boon- September 2017
Kah Hui Yap, Wei Chun Ung, Esther G. M. Ebenezer, Nadira Nordin, Pui See Chin, Sandheep Sugathan, Sook Chin Chan, Hung Loong Yip, Masashi Kiguchi
Abstract
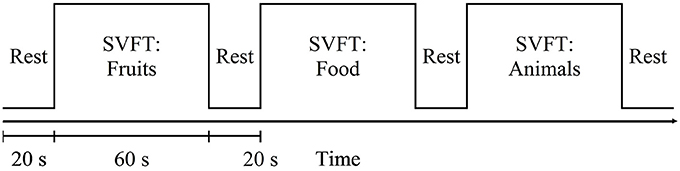
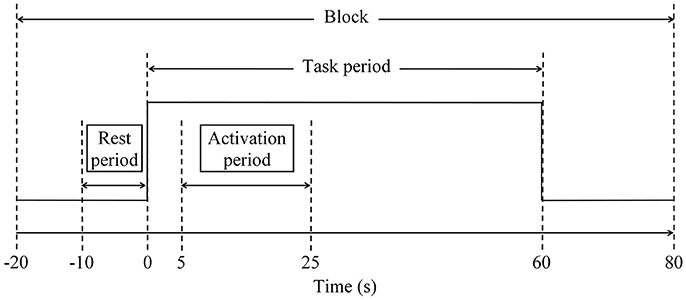
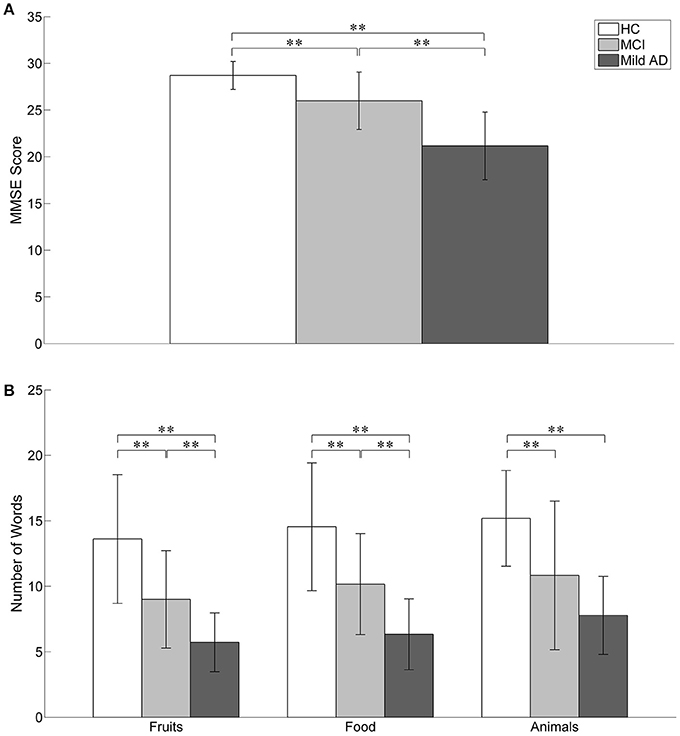
Cognitive performance is relatively well preserved during early cognitive impairment owing to compensatory mechanisms. We explored functional near-infrared spectroscopy (fNIRS) alongside a semantic verbal fluency task (SVFT) to investigate any compensation exhibited by the prefrontal cortex (PFC) in Mild Cognitive Impairment (MCI) and mild Alzheimer's disease (AD). In addition, a group of healthy controls (HC) was studied. A total of 61 volunteers (31 HC, 12 patients with MCI and 18 patients with mild AD) took part in the present study. Although not statistically significant, MCI exhibited a greater mean activation of both the right and left PFC, followed by HC and mild AD. Analysis showed that in the left PFC, the time taken for HC to achieve the activation level was shorter than MCI and mild AD (p = 0.0047 and 0.0498, respectively); in the right PFC, mild AD took a longer time to achieve the activation level than HC and MCI (p = 0.0469 and 0.0335, respectively); in the right PFC, HC, and MCI demonstrated a steeper slope compared to mild AD (p = 0.0432 and 0. 0107, respectively). The results were, however, not significant when corrected by the Bonferroni-Holm method. There was also found to be a moderately positive correlation (R = 0.5886) between the oxygenation levels in the left PFC and a clinical measure [Mini-Mental State Examination (MMSE) score] in MCI subjects uniquely. The hyperactivation in MCI coupled with a better SVFT performance may suggest neural compensation, although it is not known to what degree hyperactivation manifests as a potential indicator of compensatory mechanisms. However, hypoactivation plus a poorer SVFT performance in mild AD might indicate an inability to compensate due to the degree of structural impairment. Consistent with the scaffolding theory of aging and cognition, the task-elicited hyperactivation in MCI might reflect the presence of compensatory mechanisms and hypoactivation in mild AD could reflect an inability to compensate. Future studies will investigate the fNIRS parameters with a larger sample size, and their validity as prognostic biomarkers of neurodegeneration.
Methodology
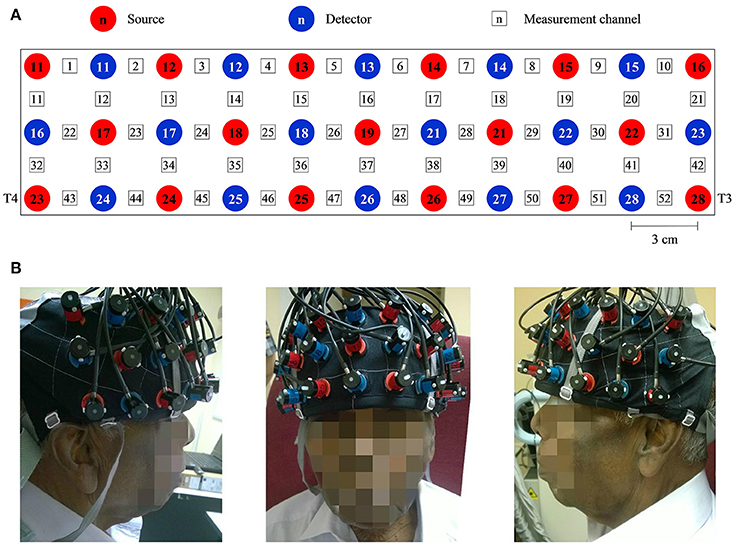
We used the Clinical Dementia Rating (CDR), an observer rating scale designed to rate the severity of dementia (Morris, 1993), for the diagnosis of dementia and group allocation. CDR scores of 0, 0.5, and 1 were assigned to HC, MCI, and mild AD, respectively; participants with CDR scores of 2 (moderate) and 3 (severe) were excluded, as this study focused on MCI and mild AD. In addition, we assessed and assigned each participant an MMSE score. CDR has a moderate to high inter-rater reliability of 0.62 (Rockwood et al., 2000). MMSE has high inter-rater reliability, ranging between 0.82 and 0.91 (Magni et al., 1996). We matched the HC to those of the combined sample of patients according to age, sex, and education.
Impact & Benefits
Improved Understanding of Pathophysiology: Hyperactivation Patterns: Identifying hyperactivation patterns in the prefrontal cortex can provide insights into the compensatory mechanisms that occur in early stages of neurodegenerative diseases. Understanding these patterns can elucidate how the brain attempts to compensate for cognitive decline. Progression Markers: Tracking changes in prefrontal oxygenation and hyperactivation can serve as markers for the progression from MCI to AD, offering a clearer picture of disease trajectories.
Early Diagnosis and Intervention: Biomarker Development: Prefrontal oxygenation patterns could serve as biomarkers for early diagnosis of AD and MCI, potentially leading to earlier and more accurate detection. Tailored Treatments: Understanding individual differences in brain activation can help in developing personalized treatment plans, targeting specific neural circuits affected in early disease stages.
Precision Medicine: Customized Care: The ability to visualize and quantify hyperactivation allows for more personalized approaches to managing and treating AD and MCI, considering each patient's unique brain activity profile.
Non-Invasive Monitoring: Safe and Accessible: Techniques like functional near-infrared spectroscopy (fNIRS) used to measure prefrontal oxygenation are non-invasive, making them safe and accessible for repeated use in both clinical and research settings.
Market Potential
Revenue Growth: The global market for Alzheimer's disease diagnostics was valued at approximately USD 3.8 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of around 10% from 2021 to 2028 . The inclusion of advanced imaging techniques can capture a substantial share of this growing market.
Adaption Rates: Non-invasive and cost-effective imaging solutions have higher adoption rates, especially in resource-limited settings. Technologies like fNIRS are relatively low-cost compared to other imaging modalities like MRI or PET scans.
Geographical Expansion: There is significant market potential in both developed and emerging markets. Developed regions, such as North America and Europe, have a high demand for advanced diagnostic tools, while emerging markets in Asia-Pacific and Latin America are witnessing increasing healthcare expenditure and awareness of neurodegenerative diseases.