Thermal degradation and pyrolysis analysis of zinc borate reinforced intumescent fire retardant coatings
Author: Faiz Ahmad - October 2018
Qandeel Fatima Gillani, M.I. Abdul Mutalib, Puteri S.M. Megat-Yusoff, Sami Ullah, Patrick. J. Messet, M. Zia-ul-Mustafa
Abstract
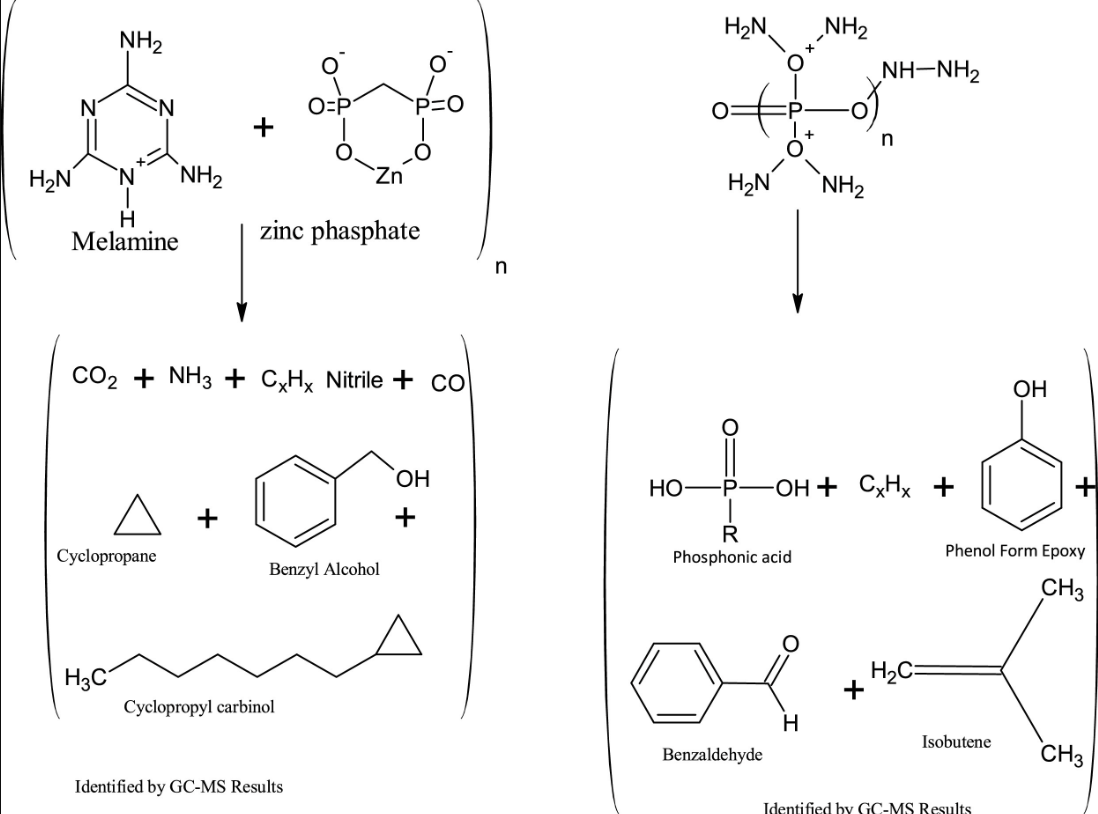
This work aims at evaluating the potential of nano-sized zinc borate as substitution of boric acid for thermal degradation and gaseous products in expandable graphite based intumescent fire retardant coating IFRC systems. The thermal degradation and pyrolysis of intumescent flame retardant coatings was characterized by bunsen burner fire test, thermogravimetric analysis (TGA), field emission scanning electron microscopy (FESEM), energy dispersive spectrometry (EDX), X-ray diffraction (XRD), fourier transformed infra-red analysis (FTIR), X-ray photoelectron spectroscopy (XPS) and gaseous emission analysis (Py-GC). Bunsen burner fire test reveals that the partial substitution of zinc borate (6.61 mass%) imparts a substantial improvement in thermal stability and reduces steel substrate temperature to 124 °C. From thermogravimetric analysis results, it was shown that this composition increases char residual mass from 39 to 46.14 mass%. The morphological structures of char residue were investigated by FESEM and it indicates that zinc borate promotes more continuous and compact char layers that hinder the heat diffusion and oxygen transmission effectively. XRD and FTIR results show that zinc borate develops a zinc-based glassy intumescent shield i.e. zinc bis (hydroxyanthrapyrimidine) dehydrate (C30H14N4O4Zn.2H2O) that strengthens the char. The new chemical species enhances the thermal stability of the freshly formed char at high temperature and provides an enhanced fire protection. XPS analysis shows the higher carbon contents in formulation IF-5 (6.6 mass%) and endorses high char residue. The Py-GC analysis confirms release of less toxic gaseous products in IF-5 formulation, considering their type and concentration, as compared to control formulation, and is considered as an environmentally safe intumescent formulation.
Methodology
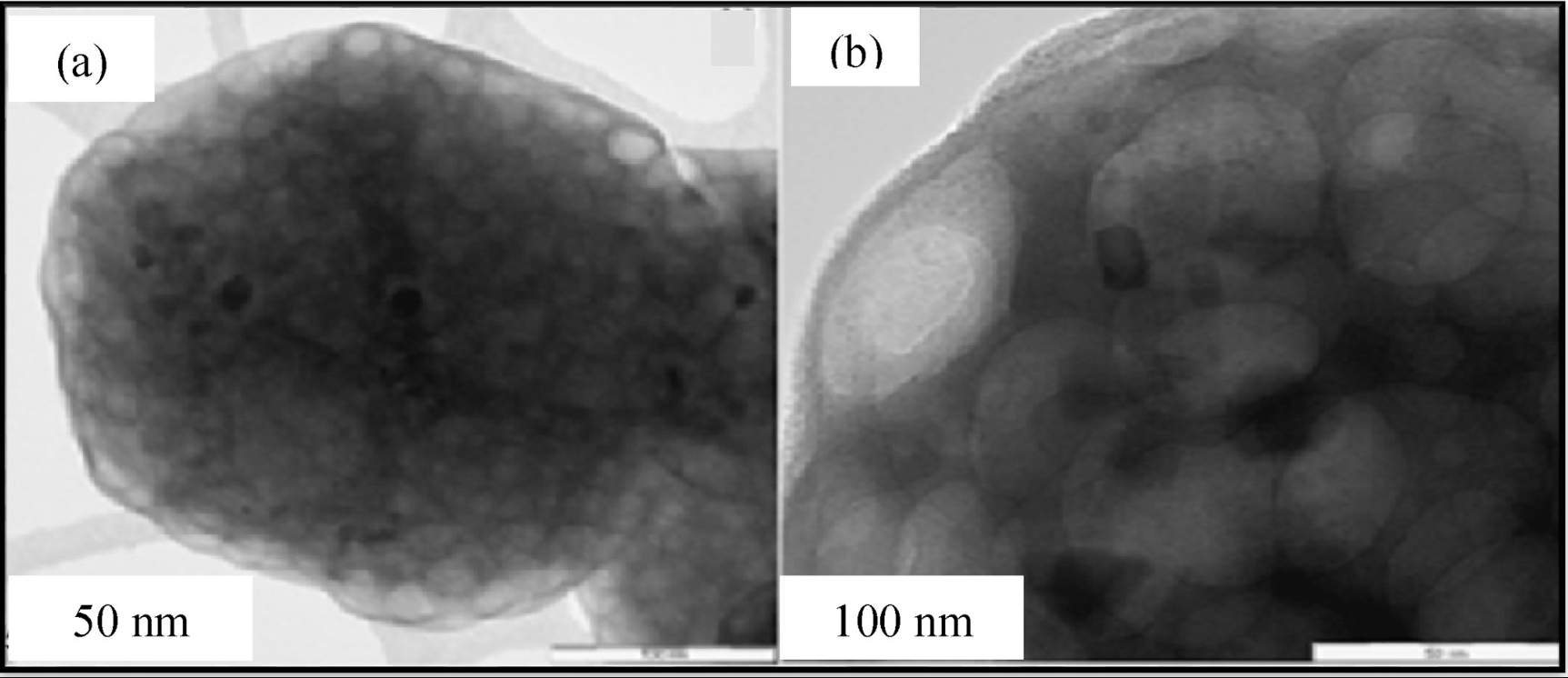
Nano-sized zinc borate, boric acid (B.A), melamine (Mel), and expandable graphite (EG), wollastonite (W) and alumina were purchased from Sigma-Aldrich (M) Sdn Bhd. Malaysia. The binder system bisphenol-A epoxy resin BE-188 (BPA) and ACR Hardener H-2310 polyamide amine were purchased from Mc-Growth chemical Sdn Bhd. Malaysia. Ammonium polyphosphate (APP) was purchased by Clariant (Malaysia) Sdn Bhd. TSA industries (Ipoh) Sdn. Bhd. Malaysia provided the structural steel A36 M. Nano-sized zinc borate used in this study was examined under transmission electron microscope (TEM). The TEM micrographs are shown in Fig. 1. The zinc borate exhibits a porous structure which can entrap blowing gases and enhance intumescence phenomena of residual char.
Impact & Benefits
Enhanced Fire Retardancy: Zinc borate improves the thermal stability of intumescent coatings, allowing them to maintain their protective char layer integrity up to higher temperatures (up to 800°C). It promotes the formation of a more compact and stable char layer, which acts as an effective thermal insulator, reducing heat transfer to the substrate.
Improved Thermal Performance: The formation of thermally stable compounds such as zinc bis(hydroxyanthrapyrimidine) dehydrate further contributes to the coating's effectiveness.
Reduction in Toxic Emissions: Zinc borate does not release toxic or corrosive elements during combustion, making it safer for use. It minimizes the emission of combustible gases and suppresses the formation of soot, leading to lower smoke generation and reduced toxicity of the emissions.
Environmental Safety: Pyrolysis analysis confirms that the zinc borate-containing formulation is safer in terms of the type and concentration of gases released during degradation.
Improved Char Formation: The release of intercalated water by zinc borate before the degradation of other components helps in forming a robust char structure, enhancing the coating's protective capabilities.
Findings/Figures and Research Data
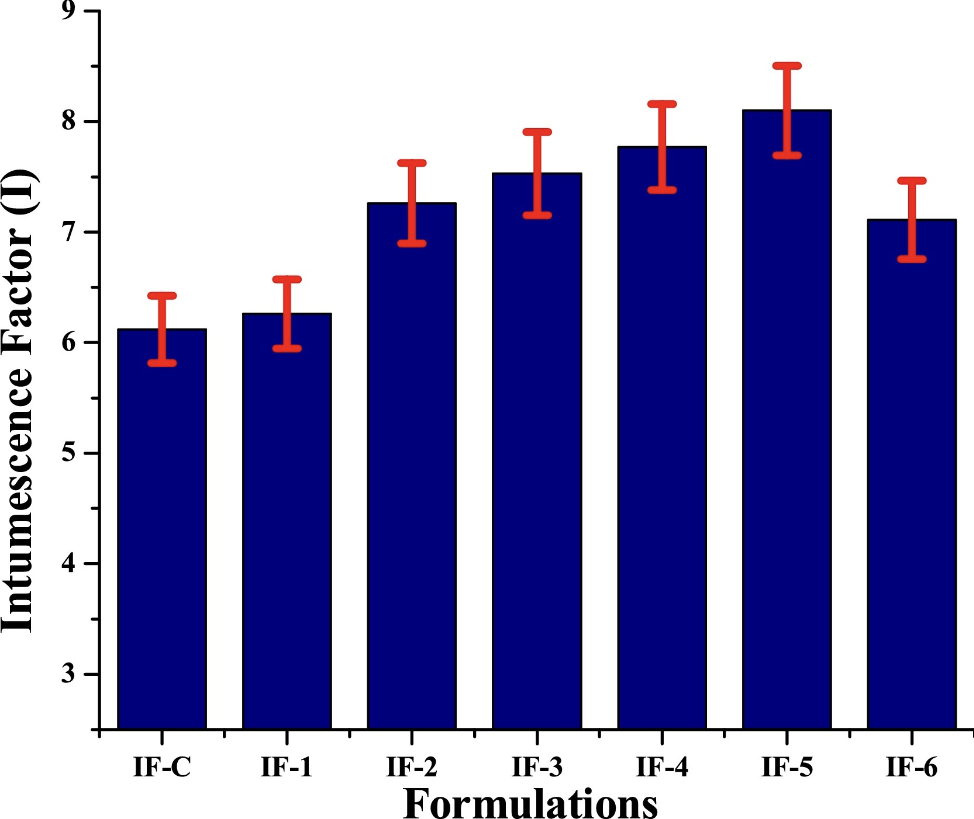
In order to compare the influence of zinc borate on the char expansion and morphology of char with control formulation, the coated substrate was burnt in the furnace. The substitution of zinc borate for boric acid in the coating formulation results in enhanced char expansion. In addition, it develops a foamy structure on the char. The efficiency of the char layer strongly depends on its physical properties such as its foam structure and expansion [36]. The thickness of the char was recorded by a digital Vernier calliper and the intumescence factor (I) was determined using Eq. (1) and presented in Fig. 4 [38].
Market Potential
Enhanced Fire Retardant Properties: Zinc borate improves the thermal stability, smoke suppression, and overall fire retardant performance of intumescent coatings. Its ability to promote the formation of a protective, cellular, and vitreous char layer enhances the heat shielding properties and integrity of the coating at high temperatures.
Reduction in Toxic Emissions:Zinc borate does not release toxic or corrosive elements during combustion, making it safer for use in various applications. It minimizes the emission of combustible gases and reduces soot formation, which can lead to lower smoke generation and reduced toxicity.
Synergistic Effects with Other Additives:The incorporation of zinc borate in combination with other flame retardant additives, such as expandable graphite, has shown synergistic effects that enhance the overall fire retardancy of the coating.
Versatility in Applications:Intumescent coatings with zinc borate can be used across multiple industries, including construction, transportation, and manufacturing, where fire protection is critical. Its ability to crosslink polymers and convert aromatic compounds into less toxic aliphatic hydrocarbons broadens its applicability.